Device Master Record Procedure Template Word
The Device Master Record Procedure Template Word is a comprehensive document that provides a step-by-step guide for creating and maintaining a device master record (DMR) for medical devices. This template is designed to help medical device manufacturers comply with the regulations set forth by the Food and Drug Administration (FDA) and other regulatory bodies.
The DMR is a critical document that contains all the information necessary to manufacture a medical device, including design specifications, manufacturing processes, quality control procedures, and labeling requirements. The Device Master Record Procedure Template Word provides a framework for creating a DMR that is accurate, complete, and up-to-date.
The template includes detailed instructions for each section of the DMR, as well as sample forms and checklists to ensure that all necessary information is included. The document is fully customizable, allowing manufacturers to tailor it to their specific needs and requirements.
By using the Device Master Record Procedure Template Word, medical device manufacturers can streamline their DMR creation process, reduce errors and inconsistencies, and ensure compliance with regulatory requirements. This can help to improve product quality, reduce costs, and enhance customer satisfaction.
Overall, the Device Master Record Procedure Template Word is an essential tool for any medical device manufacturer looking to create a comprehensive and compliant DMR. With its user-friendly format and customizable design, this template is a valuable resource for ensuring the safety and effectiveness of medical devices.
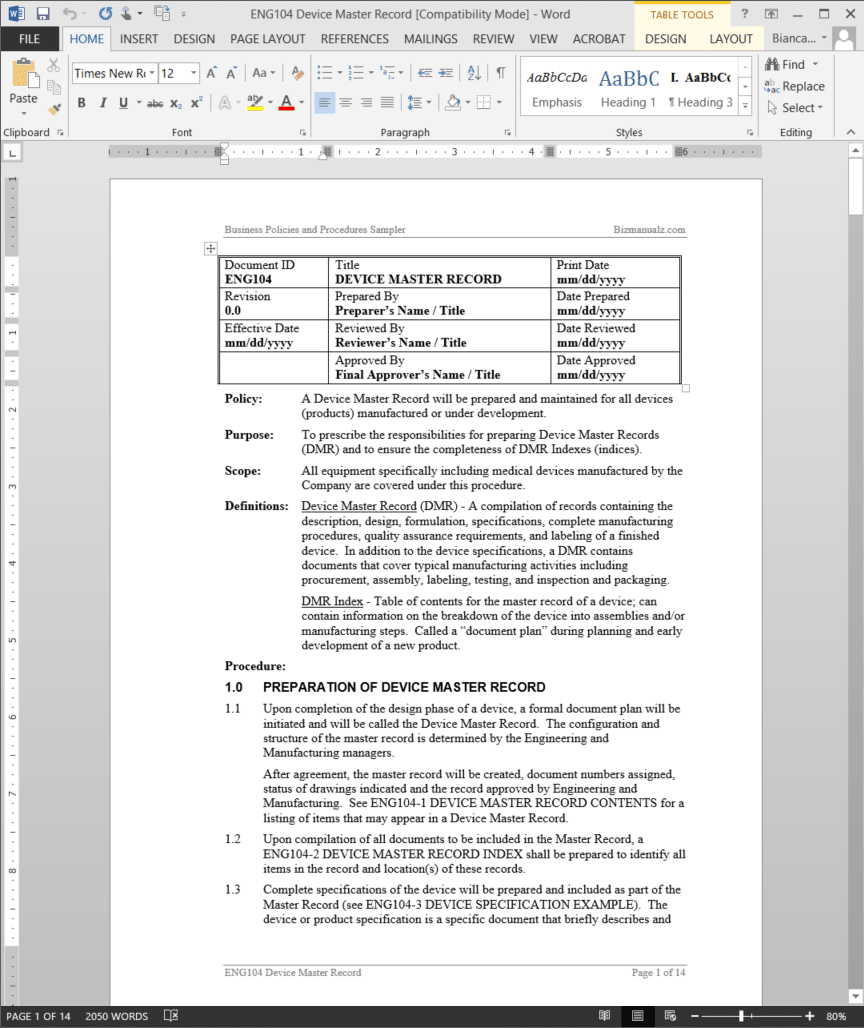
Device Master Record Procedure
The Device Master Record Procedure prescribes the responsibilities for preparing Device Master Records (DMR) and to ensure the completeness of DMR Indexes (indices).
A Device Master Record should be prepared and maintained for all devices (products) manufactured or under development. All equipment specifically including medical devices manufactured by the company are covered under the Device Master Record Procedure. (14 pages, 2,077 words)
Device Master Record Definitions:
Device Master Record (DMR) – A compilation of records containing the description, design, formulation, specifications, complete manufacturing procedures, quality assurance requirements, and labeling of a finished device. In addition to the device specifications, a DMR contains documents that cover typical manufacturing activities including procurement, assembly, labeling, testing, and inspection and packaging.
DMR Index – Table of contents for the master record of a device; can contain information on the breakdown of the device into assemblies and/or manufacturing steps. Called a “document plan” during planning and early development of a new product.
 Device Master Record Procedure Activities
Device Master Record Procedure Activities
- Preparation of Device Master Record
- Records Retention and Location
- Authorization and Change Control
Device Master Record Procedure Forms






















Reviews
There are no reviews yet.