ISO9001 2015 Calibration Record Template Template Word
The ISO9001 2015 Calibration Record Template Template Word is a comprehensive tool designed to help businesses maintain accurate records of their calibration activities. This template is specifically designed to meet the requirements of ISO 9001:2015, which is the international standard for quality management systems.
The template is easy to use and can be customized to meet the specific needs of your business. It includes all the necessary fields to record calibration activities, including the date of calibration, the equipment used, the results of the calibration, and any corrective actions taken. The template also includes a section for documenting the calibration process, including the procedures used and any deviations from those procedures.
Using the ISO9001 2015 Calibration Record Template Template Word can help your business ensure that all calibration activities are properly documented and that your quality management system is in compliance with ISO 9001:2015. This can help you improve the quality of your products and services, reduce the risk of non-conformities, and enhance customer satisfaction.
The template is available in Microsoft Word format, making it easy to use and customize. It can be downloaded instantly from the Bizmanualz website, and comes with a 60-day money-back guarantee. If you’re looking for a comprehensive and easy-to-use tool to help you manage your calibration activities, the ISO9001 2015 Calibration Record Template Template Word is an excellent choice.
ISO Calibration Record Template
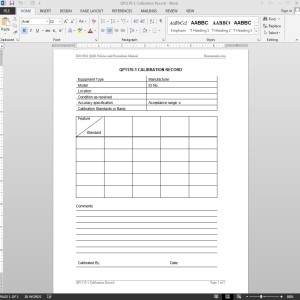
The Quality Assurance Manager should document calibrations and maintain records pertaining to calibration, repeatability, and reproductibility of data on QP1170-1 CALIBRATION RECORD. Each ISO Calibration Record Template should include, at a minimum, a unique equipment identifier (e.g., equipment name and ID), calibration date, calibration method, standard used, condition of equipment as received, any adjustments or repairs required, calibration measurement data, and identification of the person or company performing the calibration.
Typically, a calibration sticker – listing the calibration date, expiration date, instrument identification number, and initials of the person calibrating or mark of the calibrating company – is attached to the calibrated equipment. If it is impractical to attach the calibration sticker directly to the device, it should be attached to the device container or in the immediate vicinity of (e.g., kept in the same drawer/cabinet) where the equipment is kept.
It is the responsibility of everyone using measuring and test equipment to alert the Quality Assurance Manager in the event of accidental damage or any irregularity between calibrations. The Quality Assurance Manager should ensure the validity of previous measuring results against QP1170-1 CALIBRATION RECORD. Where possible, monitoring and measuring equipment must be protected from any adjustment that might invalidate the calibration and, hence, invalidate any result.
 ISO Calibration Record Template Details
ISO Calibration Record Template Details
Pages: 01
Words: 28
Format: Microsoft Word 2013 (.docx)
Language: English
Manual: Quality Assurance Policy Statement and Procedures
Procedure: ISO Control of Monitoring-Measuring Equipment Procedure QP1170
Type: Form





















Reviews
There are no reviews yet.