GMP Inspection Triggers Decision Points Guide Template Word
Ensure your organization is always prepared for GMP inspections with the GMP Inspection Triggers Decision Points Guide Template Word. This comprehensive guide provides a step-by-step approach to identifying potential triggers and decision points that may arise during an inspection, allowing you to proactively address any issues before they become a problem.
The GMP Inspection Triggers Decision Points Guide Template Word is designed to be user-friendly and easy to customize to your organization’s specific needs. The template includes a detailed checklist of potential triggers and decision points, as well as guidance on how to address each one. This ensures that your organization is fully prepared for any potential issues that may arise during an inspection.
With the GMP Inspection Triggers Decision Points Guide Template Word, you can rest assured that your organization is fully compliant with GMP regulations and prepared for any potential inspections. The guide is also a valuable resource for training new employees and ensuring that everyone in your organization is aware of the potential triggers and decision points that may arise during an inspection.
Don’t wait until it’s too late to prepare for a GMP inspection. Order the GMP Inspection Triggers Decision Points Guide Template Word today and ensure that your organization is always fully prepared for any potential inspections.
GMP Inspection Triggers Decision Points Guide Template
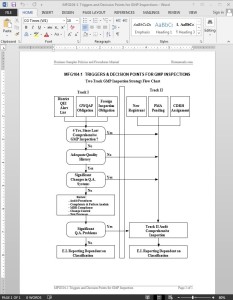
The GMP Inspection Triggers Decision Points Guide Template is a guide for the triggers and decision points for Track I and Track II GMP Inspections. Scheduling of routine “qualifying inspections” of Medical Device Manufacturers required by the FDA’s policies is generally covered by a Two Track GMP Inspection Strategy, which can be seen on MFG104-1 TRIGGERS AND DECISION POINTS FOR GMP INSPECTIONS.
Inspectional coverage under either the Track I or Track II approach will constitute a “qualifying inspection.” District management will determine which of the triggers listed apply to a firm scheduled for a qualifying inspection. All establishments that manufacture a class II and/or III device must receive a comprehensive (Track II) GMP Inspection once every four years.
Normally, district management will assist in making the tentative decision of whether the firm should be covered under Track I or II. The investigator may identify extensive deficiencies while performing a Track I Inspection which will require a more comprehensive inspection (Track II). District policy may require that investigators consult with their supervisors before initiating a more comprehensive inspection.
GMP Inspection Triggers Decision Points Guide Template Details
Pages: 01
Words: 82
Format: Microsoft Word 2013 (.docx)
Language: English
Manual: Business Sampler
Category: Manufacturing
Procedure: FDA Inspections Procedure MFG104
Type: Guide
Related Documents






















Reviews
There are no reviews yet.